FDA gives nod to first fully-removable percutaneous peripheral
$ 92.00
-
By A Mystery Man Writer
-
-
4.7(141)

Product Description
SPR Therapeutics has developed a peripheral nerve stimulation system that is placed percutaneously through the skin instead of being implanted and which can be completely removed from the body after therapy period.
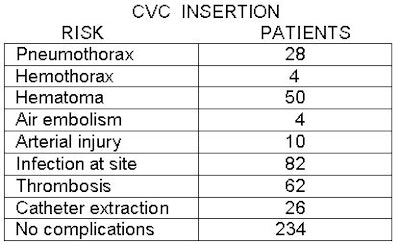
PICCing sides: Interventional radiologists weigh IV access lines
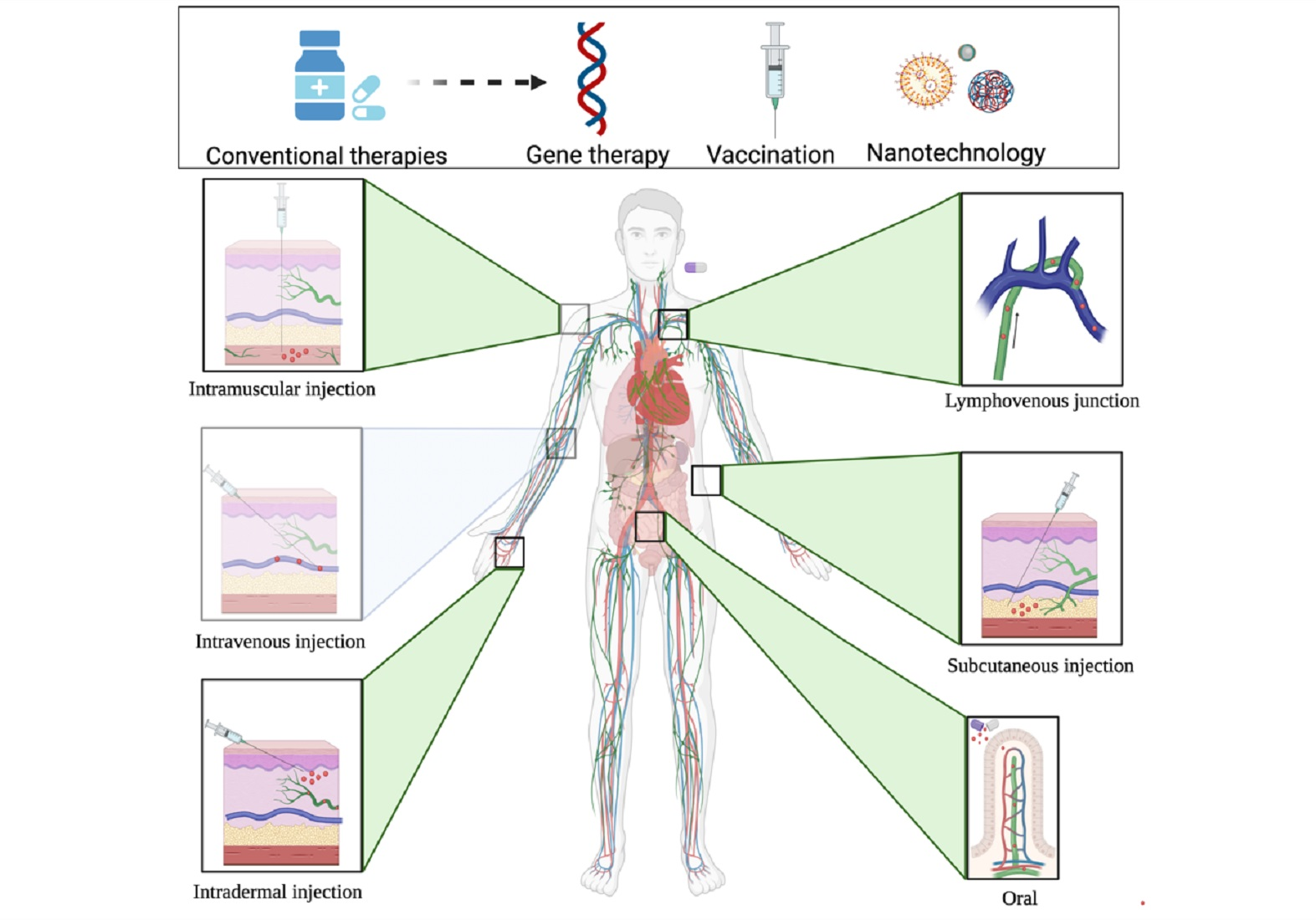
Pharmaceutics, Free Full-Text

Latest News Archives - Interventional News

AHDB March/April 2015 Vol 8 Payor's Guide by Dalia Buffery - Issuu

Renal Interventions 5 - EU by BIBA Publishing - Issuu
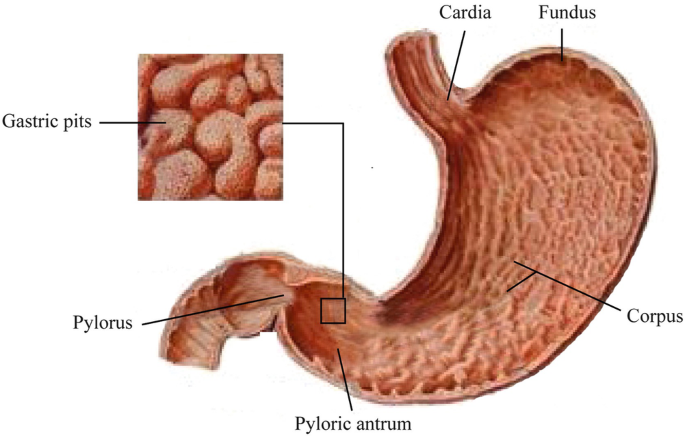
Digestive Tract Disease
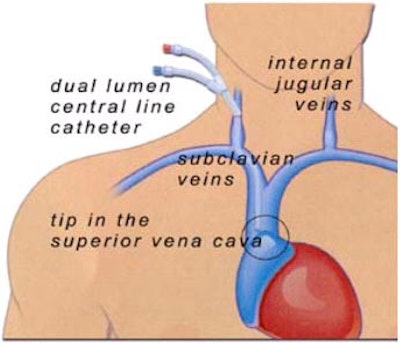
PICCing sides: Interventional radiologists weigh IV access lines

A Surgeon's Guide for Various Lung Nodule Localization Techniques and the Newest Technologies - Katie N. Cornella, Danielle C. Repper, Brian A. Palafox, Mahmood K. Razavi, Christopher T. Loh, Kelly M. Markle

PQ Bypass Receives FDA “Breakthrough Device Designation” for the World's First Fully Percutaneous Femoral-Popliteal Bypass Device
Latest News Archives - Interventional News

Latest News Archives - Interventional News
Students, Clinical Immersion Program

Recent Advances in Nanomedicines for Multiple Sclerosis Therapy






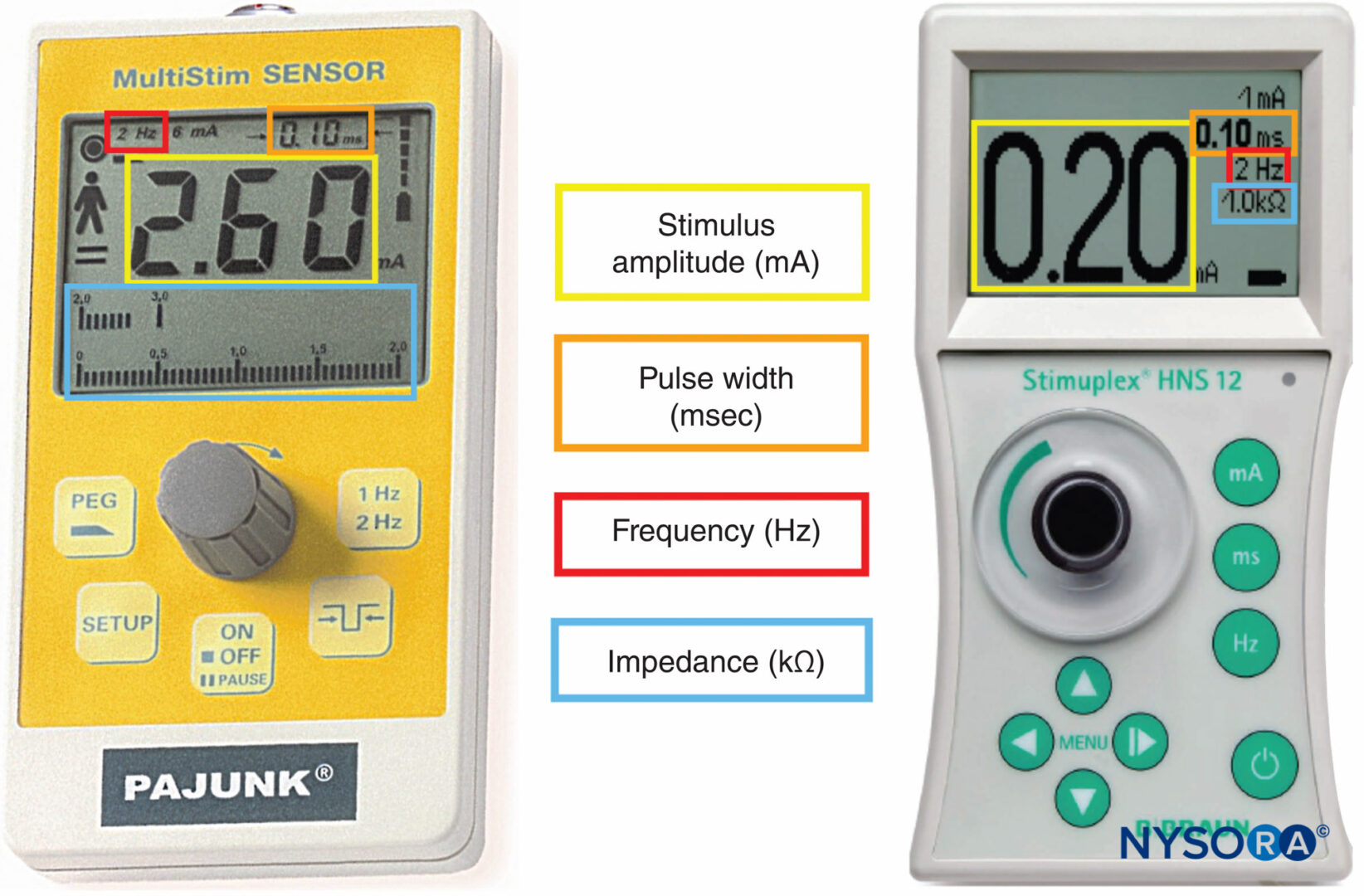


:max_bytes(150000):strip_icc()/Testing_Mr.-Coffee-Flintshire-Stainless-Steel_Fred-Hardy_0178-93605ff4581f465ab321b74ea9b675f4.jpg)





