FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood
$ 27.99
-
By A Mystery Man Writer
-
-
4.9(732)

Product Description
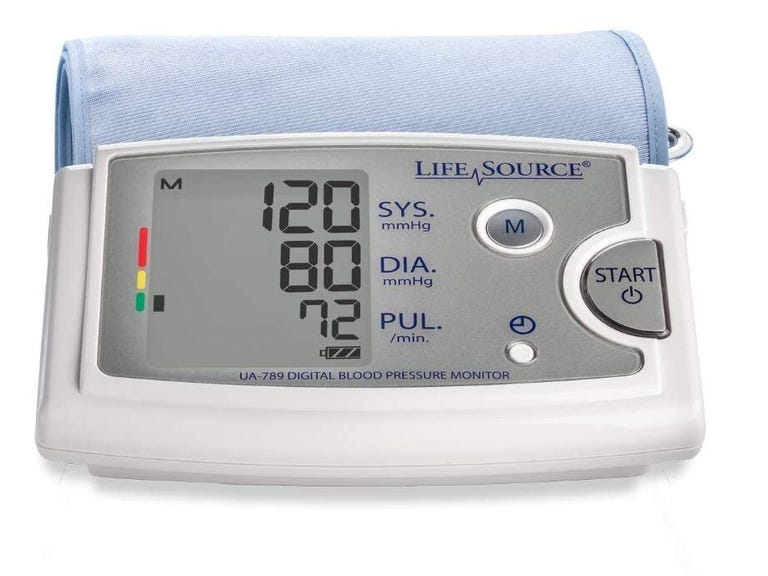
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

Mouse Archives - Inside Precision Medicine

Ep 22 Introducing Nanotechnology to improve patient outcomes - Venk Varadan - Key Tech

Multiparametric cloth-based wearable, SimpleSense, estimates blood pressure

AdvaMed (@AdvaMedUpdate) / X
Precision Medicine Informatics Articles & News - Inside Precision Medicine
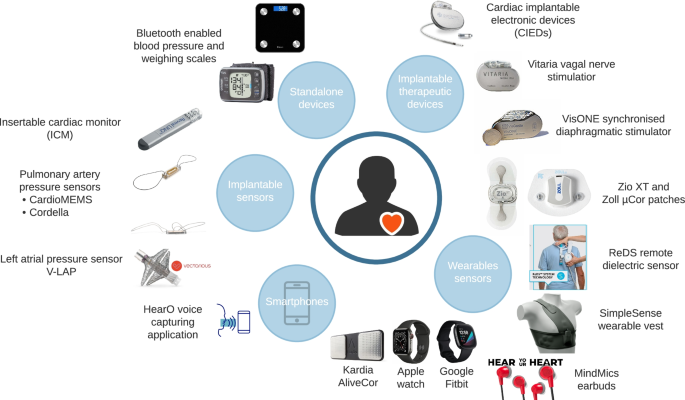
Digital Technologies to Support Better Outcome and Experience of Care in Patients with Heart Failure

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous Blood Pressure Monitoring and Hypertension Diagnostic Management: SimpleSense-BP

FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood Pressure Monitor

Nanowear's SimpleSense-BP secures US FDA 510(k) clearance
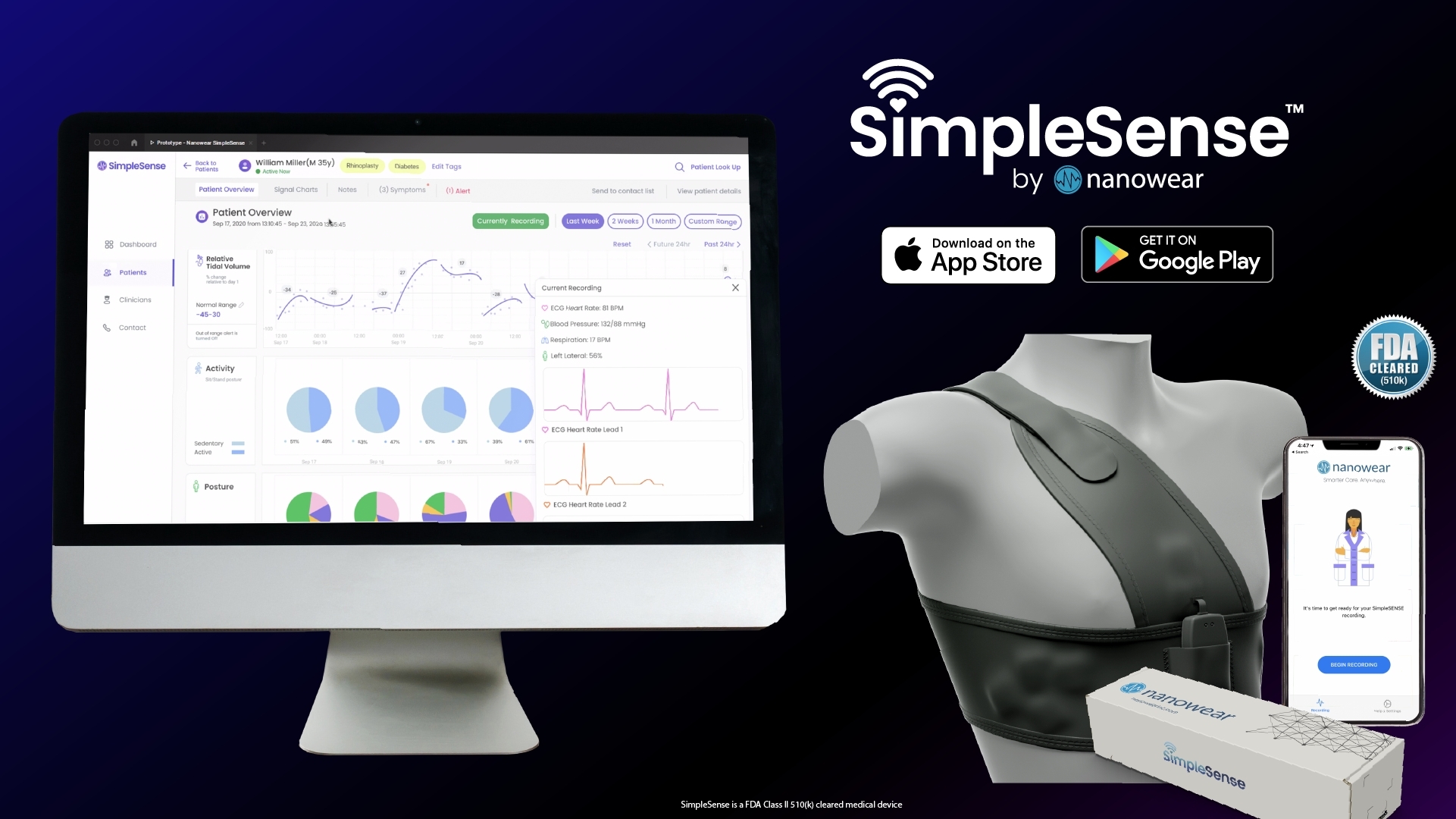
Nanowear Receives FDA 510(k) Platform Clearance to Implement Forthcoming AI-based Diagnostics in its Closed Loop Hospital-at-Home Network

Precision Medicine Precision Medicine Articles & News - Inside Precision Medicine
AI Model Uses Sequencing Data to Predict Cancer Source

Precision Medicine Informatics Articles & News - Inside Precision Medicine