FDA May Reclassify ECT Devices
-
By A Mystery Man Writer
-
-
5(413)
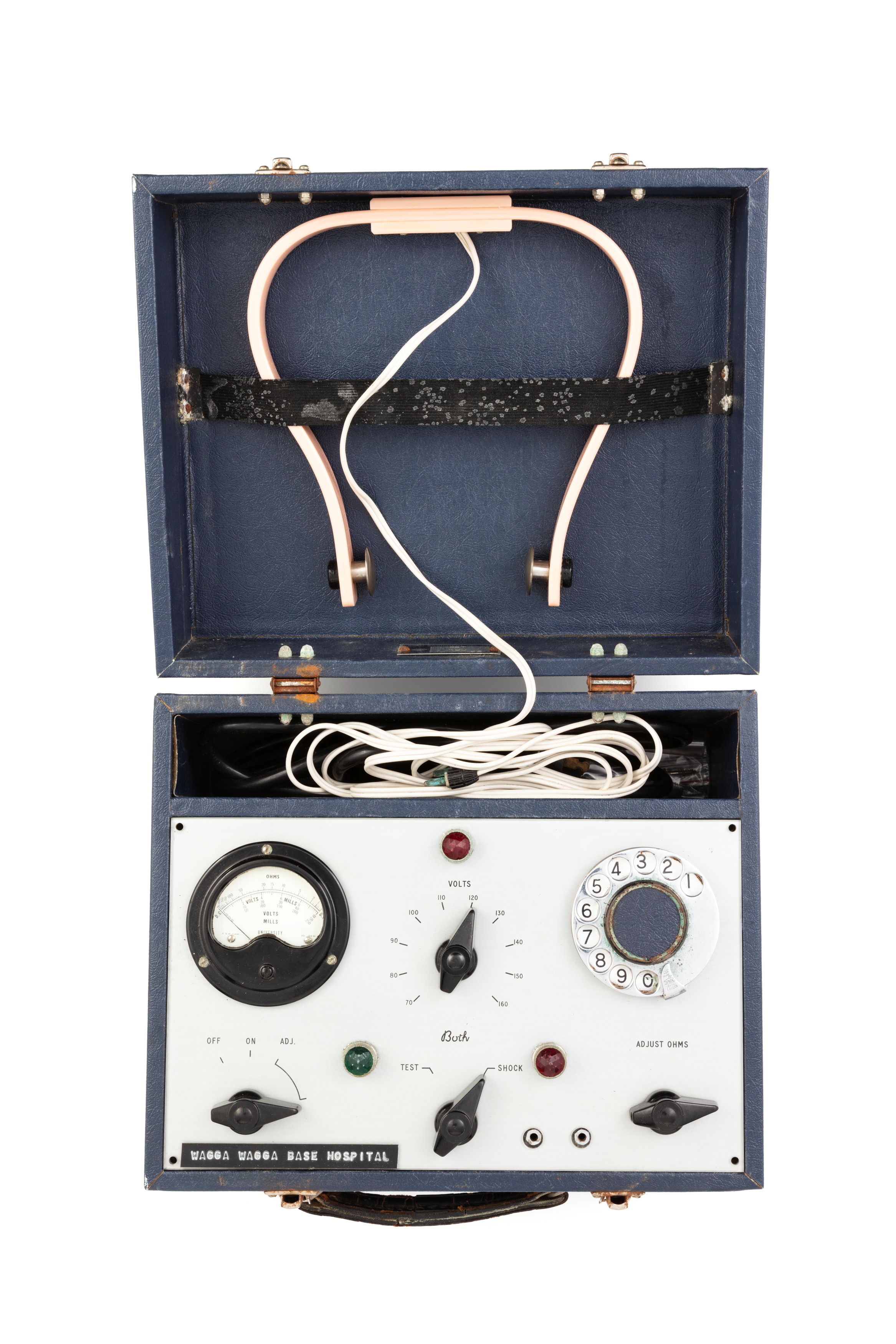
Product Description
The FDA may reclassify electroconvulsive therapy devices from class III to class II. The FDA is accepting public comments on the change until March 28.

ECT Device Manufacturer Admits Brain Damage as a Risk of

10 Facts You May Not Know About Electroconvulsive Therapy (ECT

MECTA Electroshock Device Manufacturer Files for Bankruptcy as its

FDA Looks to Expand Electroshock Use Despite Significant Risks and
Key updates in the clinical application of electroconvulsive

FDA Downgrades Cranial Electrotherapy Devices To Class II

ECT: Dangerous on Either Side of the Pond
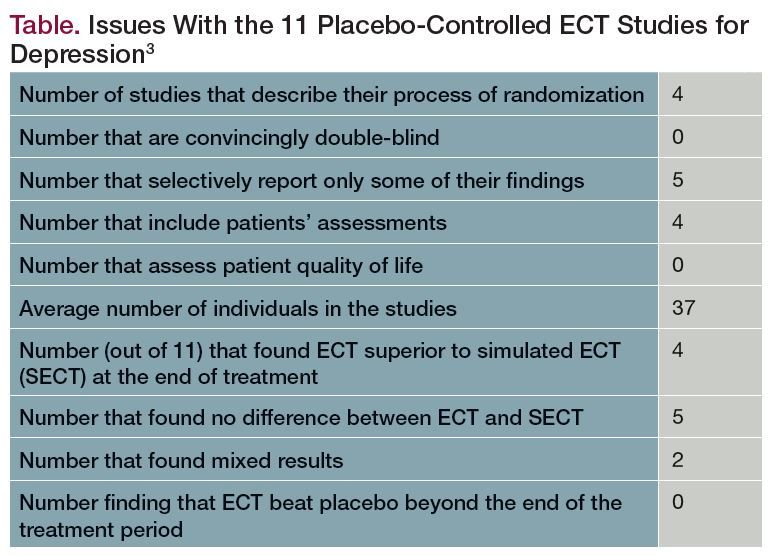
ECT: Dangerous on Either Side of the Pond