FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood
-
By A Mystery Man Writer
-
-
4.5(164)

Product Description
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

Multiparametric cloth-based wearable, SimpleSense, estimates blood
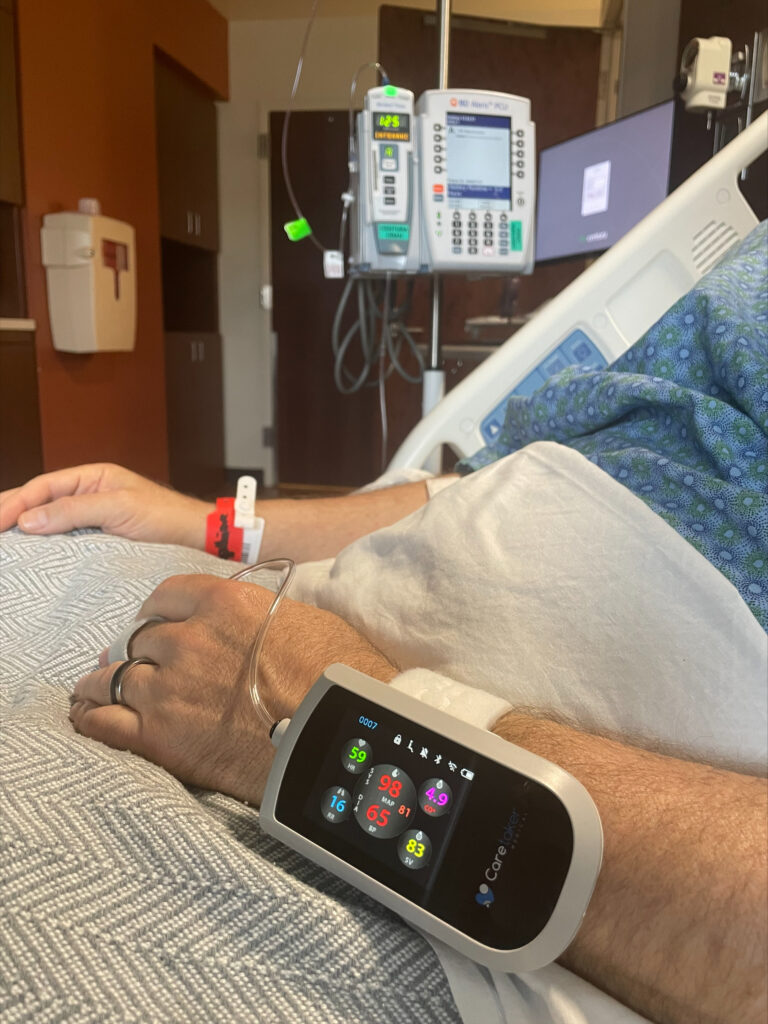
Caretaker Medical's Wireless Monitor is FDA Cleared
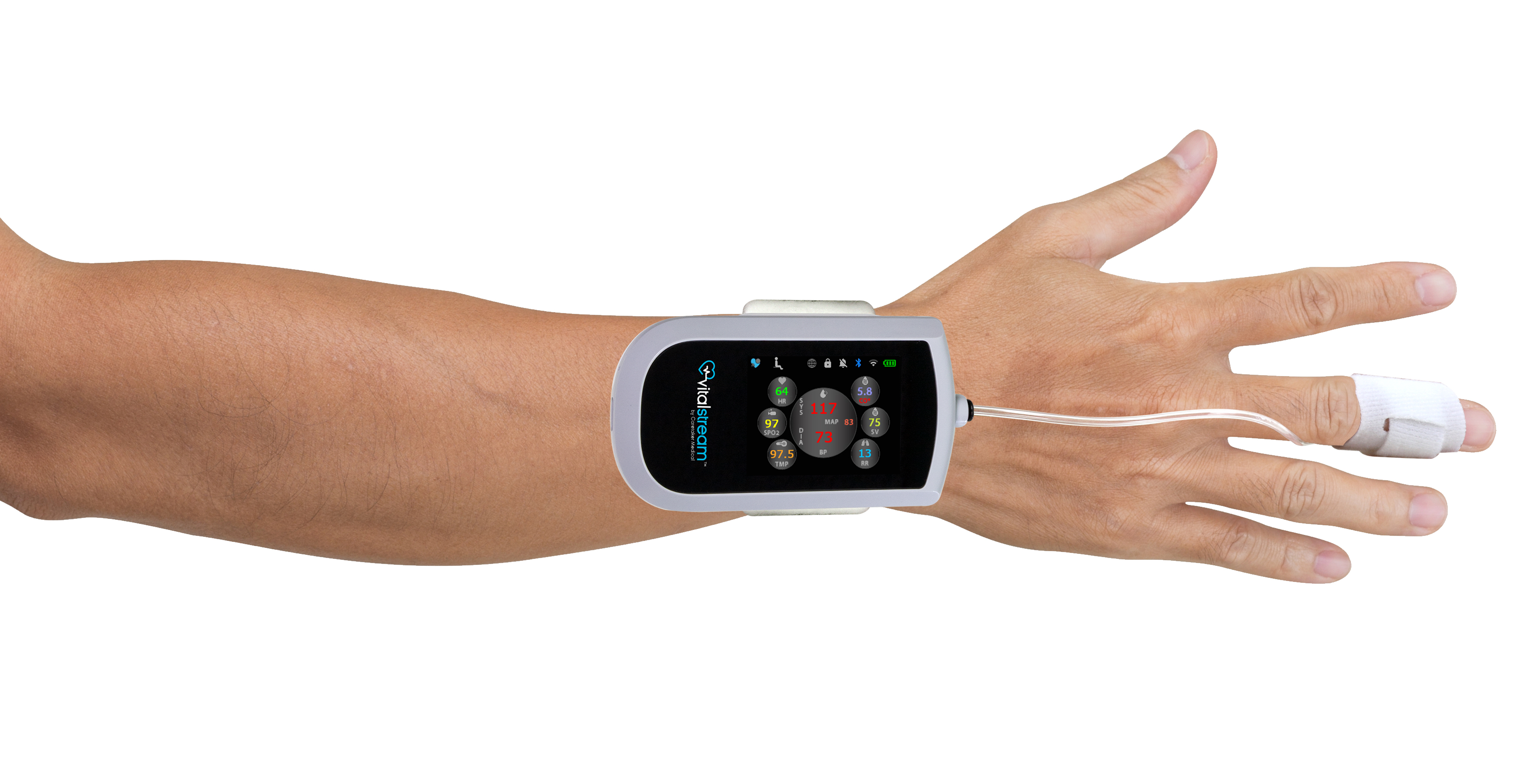
FDA Clears Caretaker Medical's Wireless Platform for Continuous
Experimental Gene Therapy to Reverse Common Hearing Loss Has Early

Researchers File Patent for Tiny Blood Flow Sensor

Precision Medicine News & Precision Therapy Features

FDA Clears Caretaker Medical's Wireless Monitor for Continuous
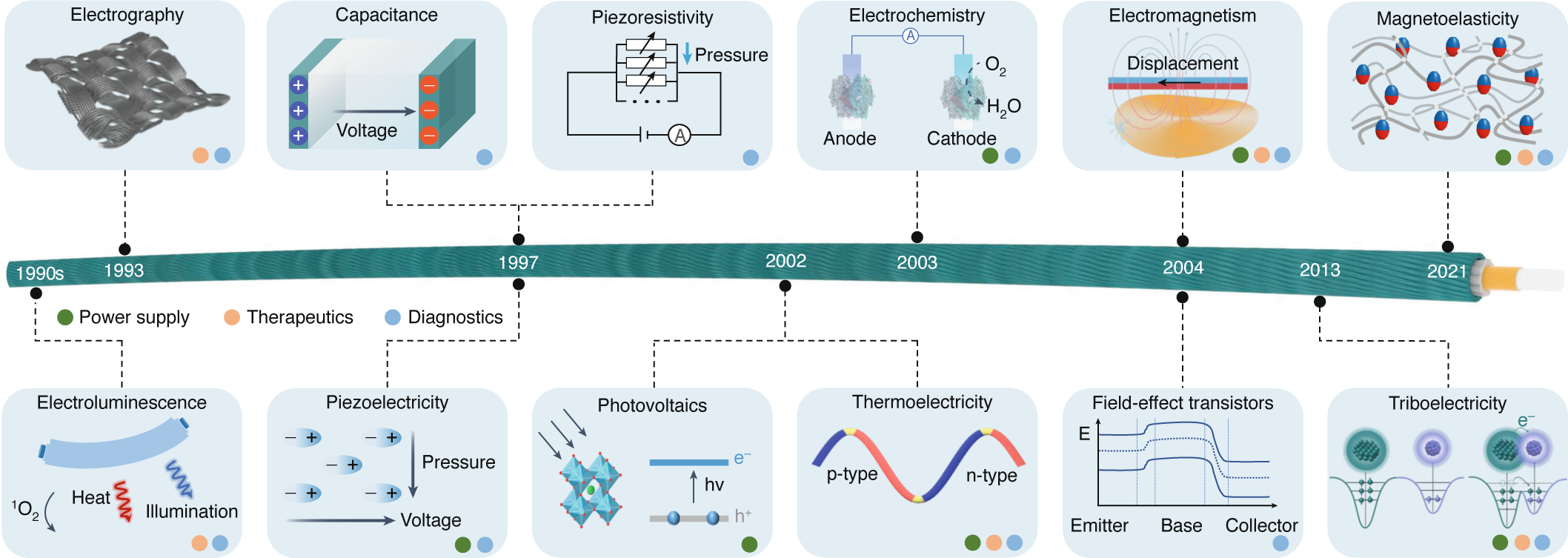
Smart textiles for personalized healthcare















